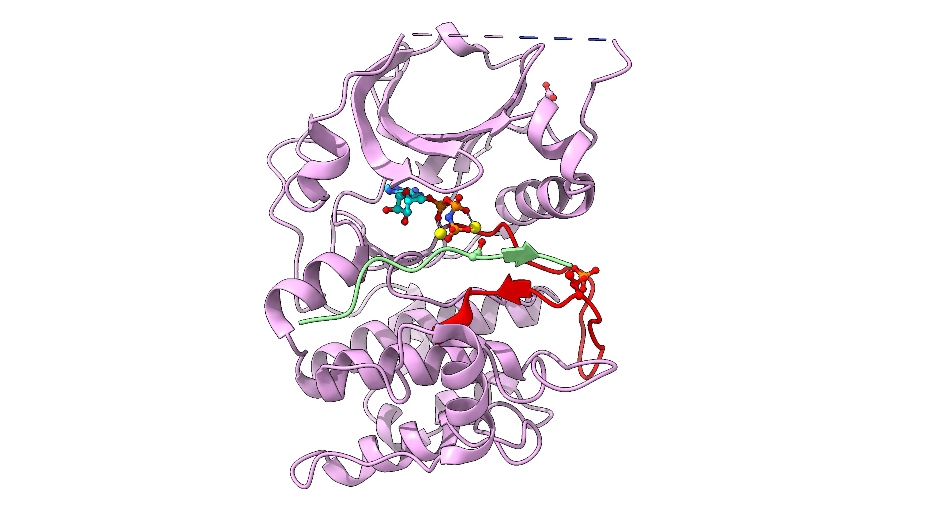
Image: Cartoon representation of AKT2 (PKB beta) kinase domain. Kinase domain in pink, activation segment in red, GSK3 substrate peptide in light green. The phosphorylated Thr309 residue (activation segment) and Asp474 (phosphoSer474 mimic), and ATP-Mn are shown. Credit: David Barford
FDA approval for capivasertib
Today is a day of great celebration here at The Institute of Cancer Research. The FDA has approved the breast cancer drug capivasertib, which was discovered following a major, long-running research programme at the ICR with partners including Astex Pharmaceuticals and AstraZeneca.
Capivasertib’s FDA approval follows on from positive trial results last year and means that health providers in the US can now begin prescribing the drug to patients, bringing it to one of the world’s largest patient populations.
We hope that capivasertib, which has now also been given the brand name Truqap, will benefit many thousands of patients with breast cancer in the US and that – as is often the case after FDA approvals – the drug will also soon be approved by other regulators, including in Europe, and become available for patients here in the UK.
Patient impact through industry collaboration
The ICR is one of the world’s most successful and influential cancer research institutions. We publish the results of hugely impactful science from across the full spectrum of cancer science every week, run dozens of clinical trials at once with our hospital partner The Royal Marsden, and currently work with more than 100 industry partners to drive our discoveries towards patient benefit.
We are the second-rated higher education institution in England for research quality, impact and environment according to the UK Government’s REF assessment, and the best in biological sciences.
Nevertheless, today is one of our most exciting of the past decade. It’s an especially rewarding one for all the researchers involved in its discovery.
Partnering with the ICR
Capivasertib’s FDA approval is also a reminder of how working with the ICR can lead to major successes for our industry partners. We have several oncology projects in our pipeline now for which we are seeking partners, including in drug discovery and development – and with the right partner, that could go on to replicate capivasertib’s success.
Our therapeutic pipeline
This graphic shows the ICR’s cancer drug pipeline.
We’ve played a leading role in the discovery and development of all these cancer drugs. In most cases, we ‘discovered’ the final drug molecules – in the scientific sense, meaning we created and synthesised them, in the research group now known as the Centre for Cancer Drug Discovery.
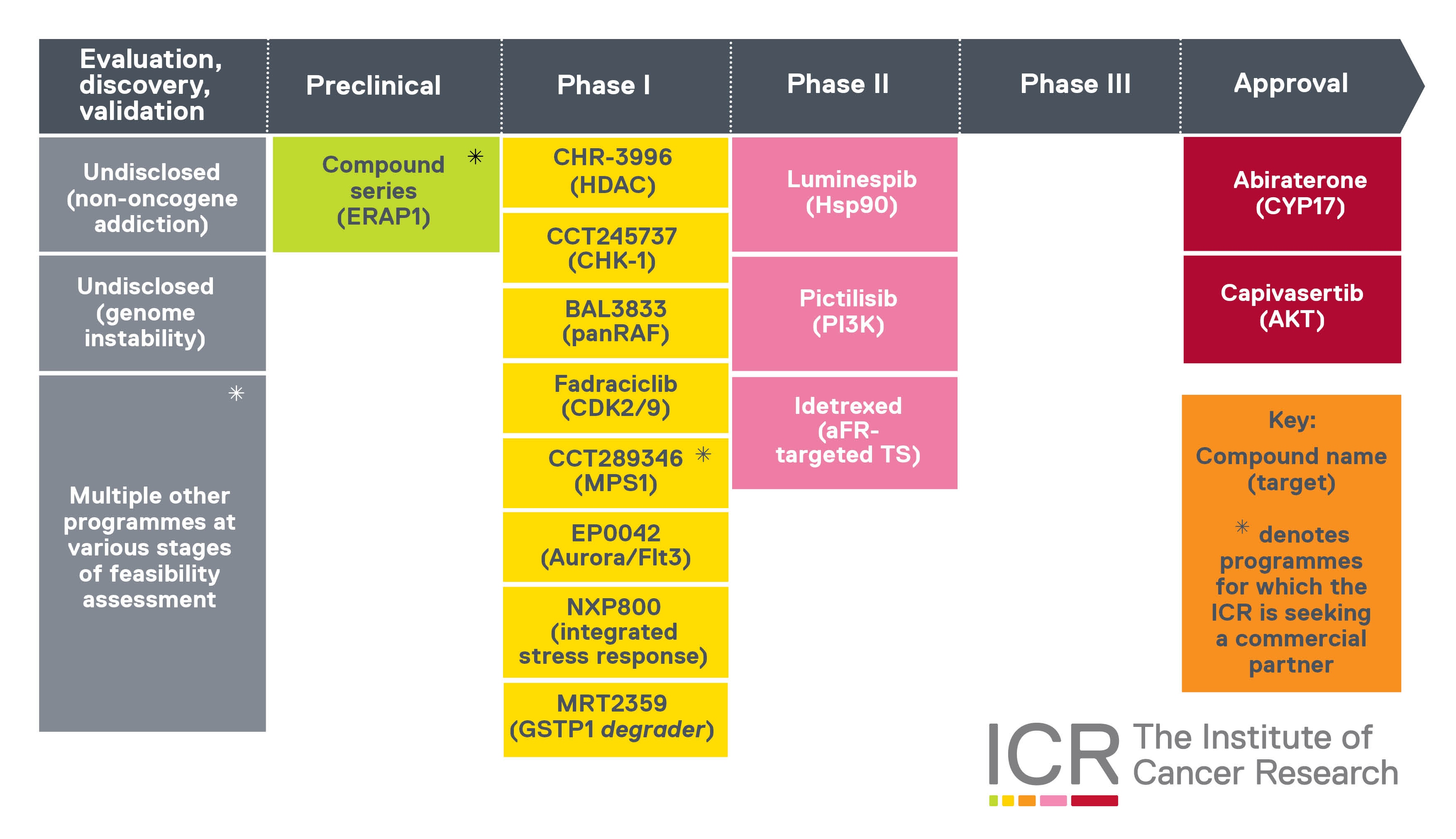
For some of these cancer drugs – like capivasertib or Monte Rosa Therapeutics’ molecular glue protein degrader MRT-2359 – our research generated precursors or prototypes that were adapted into final iterations by our partners.
And not only have we played a leading role in the discovery of these drugs. We’ve also carried out pioneering work to visualise and understand the targets of them, discovered and validated various types of biomarkers – as used in the Pharmacological Audit Trail – tested them in preclinical cancer models, developed imaging tests for them, invented platform technologies, run early-stage clinical trials, and more.
New partnering opportunities in oncology
We’re currently promoting around 15 opportunities for industry partners in oncology to collaborate with our researchers.
See all opportunities
Projects include a Centre for Cancer Drug Discovery project to further develop a lead-stage series of potent, orally available small molecule inhibitors of human ERAP1, and to further develop a phase I clinical trial stage inhibitor of the monopolar spindle assembly checkpoint protein MPS1.
Other opportunities include partnerships and licenses in MedTech including imaging, digital pathology, biomarkers to predict the effectiveness of cancer drugs, and advanced therapies including projects to develop a CAR-T therapy and a potential cancer vaccine.
Any of these opportunities could, like capivasertib, go on to become approved cancer treatments used around the world. If you’re interested in working with our researchers, get in touch with the Business Development Managers listed in the brochures or email [email protected].
Collaboration opportunities
comments powered by