Credit: Co-first author Dr Iwo Kucinski, University of Cambridge
Scientists have found a way to monitor in real time how bone marrow stem cells produce the different types of cells in the blood – a process called haematopoiesis.
After creating a new mouse model to capture real-time cell behaviour and the associated gene expression patterns in high resolution, the researchers were able to develop a clear picture of how haematopoietic stem cells (HSCs), which reside in the bone marrow, behave.
The ability of the mouse model to capture in vivo real-time cell behaviour in relation to gene expression patterns means that it can be used to explore any aspect of bone marrow haematopoiesis, in both physiology and disease.
This new approach represents an important contribution to cancer research because the abnormal differentiation of HSCs can lead to leukaemia, lymphoma and myeloma – the main types of blood cancer. It can also affect tumour progression. Therefore, it is crucial that scientists can understand and even predict how these cells respond to changes in their natural environment, such as in the bone marrow.
Researchers at The Institute of Cancer Research, London, co-led the study, which has been published in the journal Cell Stem Cell. The collaborative work from the Göttgens, Kranc and O’Carroll groups was carried out at the Queen Mary University of London, the University of Cambridge and the University of Edinburgh, and it was funded by Cancer Research UK, Wellcome, Barts Charity and Blood Cancer UK.
A significant advance
HSCs are defined by their ability to self-renew and their potential to differentiate into any of the main types of cells in the blood: T-lymphocytes, B-lymphocytes, red blood cells (erythrocytes), platelets, basophils, eosinophils, neutrophils or monocytes.
These stem cells progress through various intermediate stages during maturation, generating different types of progenitor cells that mature into cells of distinct lineages. For instance, some HSCs produce lymphoid progenitor cells, which go on to become T-lymphocytes and B-lymphocytes.
Scientists have known about this haematopoietic hierarchy for many years, but until now, they have faced obstacles when attempting to model the progression of individual cells through it in real time – something that is only possible in a living organism’s bone marrow.
Immunophenotyping, a type of test used to detect the presence of blood cell markers, provides only low-resolution snapshots of blood production and cannot distinguish between different haematopoietic stem and progenitor cells. Meanwhile, an alternative technique called single-cell RNA sequencing (scRNA-seq), which reveals the gene expression of individual cells in a given population, does not include a temporal element, so it cannot clearly portray how these cells change over time.
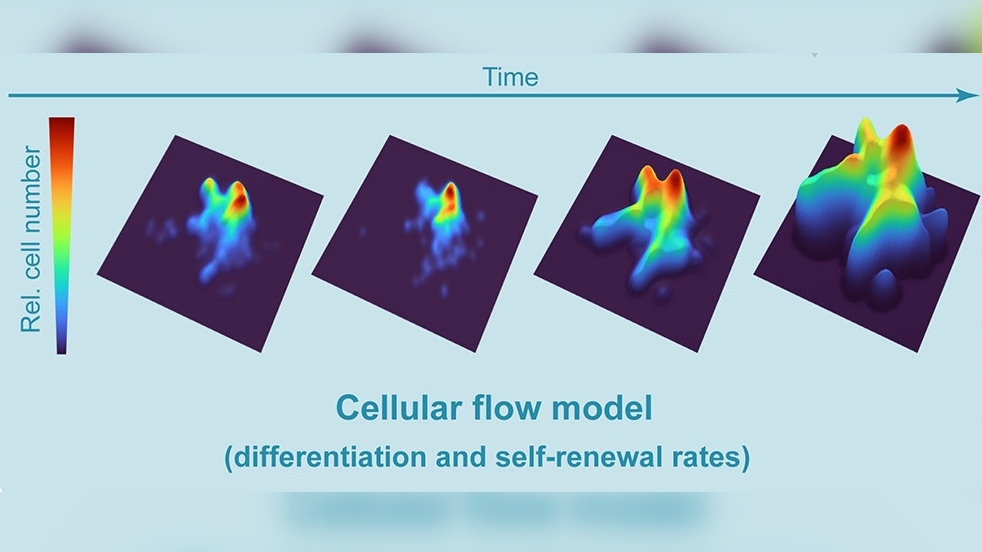
To overcome these issues, the researchers behind the current study developed a new mouse model. They induced the expression of a reporter gene in HSCs, which genetically labels them and makes it possible to track their development into differentiated cells. They then carried out scRNA-seq at various time points to determine the dynamic relationships between stem cells and progenitor cell populations, including the rates of cell division and the time taken for cells of different types to mature. Notably, based on these findings, the researchers used mathematical modelling to develop a predictive framework for further in vivo experiments.
New insights into blood cell production
By comparing the number and type of labelled cells in each scRNA-seq analysis, the team was able to precisely calculate blood cell differentiation rates – something that has not been achieved before. A high number of labelled progenitor cells in a downstream cell compartment was indicative of a fast transition between stages of maturation, whereas a lack of change between analyses suggested high rates of HSC/progenitor cell self-renewal instead.
One of the many specific questions that the researchers wanted to answer was whether common myeloid progenitor cells (CMPs) can differentiate into any of the myeloid cell types – erythrocytes, platelets, neutrophils or monocytes. Researchers have mixed views on this, with some stating that most CMPs are in fact biased towards producing a particular cell type, meaning that they are not multipotent.
The study, co-led by Professor Kamil Kranc – the ICR’s new Director of the Centre for In Vivo Modelling, showed that CMPs can only produce different types of differentiated cells in the right conditions. When the CMPs were transplanted or surrounded by cytokines – signalling proteins that affect cell growth – they tended to generate only cells of the same type. However, when given more time to expand in their native environment of the bone marrow, the CMPs proved to be multipotent.
Professor Kranc, Professor of Haemato-Oncology at the ICR, said:
“Our analyses showed that progenitor cells such as CMPs require sufficient time to ‘explore’ their potential fates and give diverse blood cell outputs. However, when conditions promote rapid differentiation, progenitor cells have insufficient time to decide their fate choice, and they are channelled towards producing a less diverse output.”
The researchers also studied how gene expression changed alongside certain cell behaviours, focusing on cells that were undergoing increased proliferation or accelerated differentiation. They identified specific patterns of gene expression that correlated with cell self-renewal and the development of certain types of blood cells.
Providing a novel framework for further research
This new approach could help researchers worldwide get a better understanding of how different conditions, such as chemotherapy, ageing and infection, affect the dynamics of blood cell production in cancer and other diseases.
Professor Kranc, who joined the ICR from the Queen Mary University of London, said:
“How HSCs function in situ in the bone marrow and how they generate diverse progenitors in real time during unperturbed haematopoiesis has never been resolved. Our paper has directly addressed this.
“What’s more, our approach has the potential to reveal novel biological dynamics at a high resolution in any biological system. We hope that other researchers can also apply it to understanding these dynamics in other tissues and organs, including during cancer.
“Mouse models are one of our most valuable tools in cancer research because they allow us to study tumours with the same characteristics as human tumours, giving us better data than we can get using cancer cells in a laboratory dish. Our new and vast datasets are available to the research community, and we believe that our innovative model can be employed to predict how normal and malignant haematopoiesis can respond to different stimuli in physiology, in disease and upon treatment.”