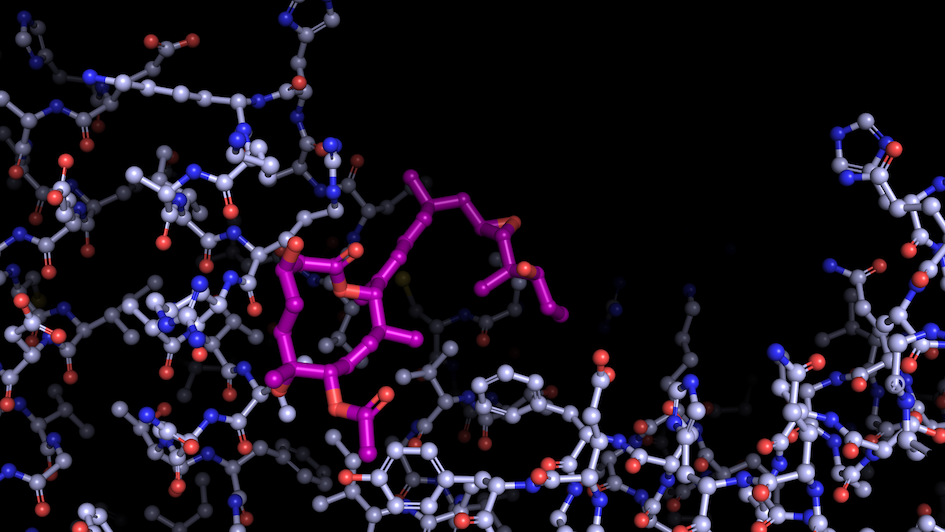
Image: “Like a constellation of atoms” – splicing modulator (purple) bound to a spliceosome subunit. Credit: Professor Vlad Pena.
Deciding what we need in our lives and what to cut out is no simple task. When it comes to our cells, this responsibility lies with the spliceosome.
The spliceosome is arguably the most complex macromolecular machine, and certainly one of the biggest and most dynamic in the nucleus. Its complexity matches the difficulty of its task.
This task is to transform the genetic information held in RNA molecules – short-living copies of the genes stored in cells as DNA. The spliceosome stitches together ‘coding’ sequences of this RNA (called exons) in a combinatorial fashion to produce the so-called messenger RNA, or mRNA. The mRNA goes on to build the proteins that make our bodies function.
Consequently, several mRNA copies will result from the same gene, that later will be translated into more types of proteins. By increasing the diversity of proteins, spliceosomes play a vital role in enhancing the complexity of organisms.
The other side of the coin is that malfunction of splicing is a hallmark for diseases such as cancer. Scientists hope that decoding splicing can help understand how healthy human cells function and how to better treat diseases resulting from mutations.
Splicing’s role in life and disease
 Vlad Pena, our Professor of Structural Biology and Gene Expression, leads a research programme focusing on splicing and its implication for diseases. His team uses biochemistry and cryo-electron microscopy (cryo-EM) to examine how splicing is regulated and how it communicates with other gene expression processes.
Vlad Pena, our Professor of Structural Biology and Gene Expression, leads a research programme focusing on splicing and its implication for diseases. His team uses biochemistry and cryo-electron microscopy (cryo-EM) to examine how splicing is regulated and how it communicates with other gene expression processes.
Professor Pena recently joined the ICR from the Max-Planck Institute of Biophysical Chemistry in Goettingen, Germany, where he first began studying the spliceosome during his postdoctoral research. At that time, researchers mainly used x-ray crystallography to study the structures of subunits rather than the intact spliceosome. The spliceosome had remained on crystallographers’ ‘most wanted’ list for decades, but its size and dynamic behaviour made it impregnable.
Over the last several years, structural biology has been transformed by the rise of electron cryo-microscopy (cryo-EM). This type of microscopy exposes samples at extremely low temperatures to a beam of electrons to study the structure of molecules. Using cryo-EM, experiments that took years with crystallography can now yield results in months.
This method has given us a 3D snapshot of the spliceosome during key functional stages of splicing and at near-atomic resolution.
Cross-talk in the cell nucleus
Scientists now have a better picture of the spliceosome but there is still much to explore. For Professor Pena, the next frontier in splicing research is to capture ‘cross-talk’ within the cell nucleus.
The chemistry of splicing is fairly straightforward, but the splicing machinery is very complex, suggesting it is involved in much more than we currently understand. During the splicing process, the spliceosome interacts with many other parts of the cell. The Pena lab aims to capture these interactions between splicing and other processes within the nucleus.
Professor Pena says the ICR provides the ideal environment to advance this research. A bioreactor was recently installed here for producing human cells in large amounts for structural studies, and a new state-of-the-art cryo-EM microscope is now operational. Researchers can also access shared microscopes at the Francis Crick Institute.
Most importantly, the ICR is home to leading scientists across disciplines and stages from the lab to the clinic. This is the perfect match for the investigation of a group of molecules called modulators – an important research topic in the Pena lab. These bind spliceosomes and regulate the splicing of specific bits of code enscribed in the RNA, rather than blocking the process entirely, which has helped his team to capture the spliceosome’s inner workings.
As these modulators have anti-tumour properties, Professor Pena aims to develop new potential cancer drugs, in close collaboration with scientists from the ICR’s Division of Cancer Therapeutics.
The universe in a molecule
Splicing remains one of the most intricate cellular processes, with a level of coordination between atoms that scientists are still trying to grasp. Professor Pena says his research has always been driven by a desire to understand the complexity of life at the atomic level.
He recalls a time in high school when he saw an image of a myoglobin molecule in the Lehninger textbook, with individual atoms and bonds in a three-dimensional structure rather than as a chemical formula.
Professor Pena says: “That breathtaking constellation of atoms gave me a flash of insight. A sudden realization that an entire universe of charming entities, behaving by rules dictated by physics and chemistry, lies behind living organisms. Such a wonder and so much to explore.”
Now he dedicates his efforts for revealing some of these marvels – macromolecular assemblies of atoms that regulate the inner workings of our cells.
comments powered by