Regulation and molecular mechanisms of tankyrase
ADP-ribosylation is a post-translational modification carried out by ADP-ribosyltransferases (ARTs), which transfer ADP-ribose from NAD+ onto substrates. ADP-ribosylation controls many aspects of cell function, including DNA repair, cell division, telomere maintenance, chromatin dynamics, apoptosis and various signal transduction processes. Given their roles in DNA repair, telomere homeostasis and cancer-relevant signalling pathways, several ARTs are being explored as potential cancer therapy targets.
In humans, the family of intracellular ARTs encompasses 17 members with similar catalytic domains but greatly diverse non-catalytic accessory domains. Different catalytically active ARTs can either transfer a single unit of ADP-ribose or attach ADP-ribose processively, thereby constructing poly(ADP-ribose) (PAR) chains, which can be of varying length and structure. Enzymes in the latter group are known as poly(ADP-ribose)polymerases (PARPs). Compared to other types of post-translational modification, such as phosphorylation, PARylation remains understudied.
We take a particular interest in the PARP enzyme tankyrase, which fulfils a wide range of biological functions, many of which are relevant to cancer. The human genome encodes two highly similar tankyrase paralogues, TNKS and TNKS2. Both share a C-terminal catalytic PARP domain, a set of five N-terminal ankyrin repeat clusters (ARCs) responsible for substrate recruitment, and a polymerising sterile alpha motif (SAM) domain in between.
Our previous structure-function work has revealed the mechanisms of substrate recognition and polymerisation by tankyrase and shown that tankyrase can act as a scaffolding protein, independently of its catalytic function. We now aim to use both X-ray crystallography and cryo-electron microscopy to understand how tankyrase’s various domains act together. Moreover, we work with chemists to develop novel approaches to modulate tankyrase function.
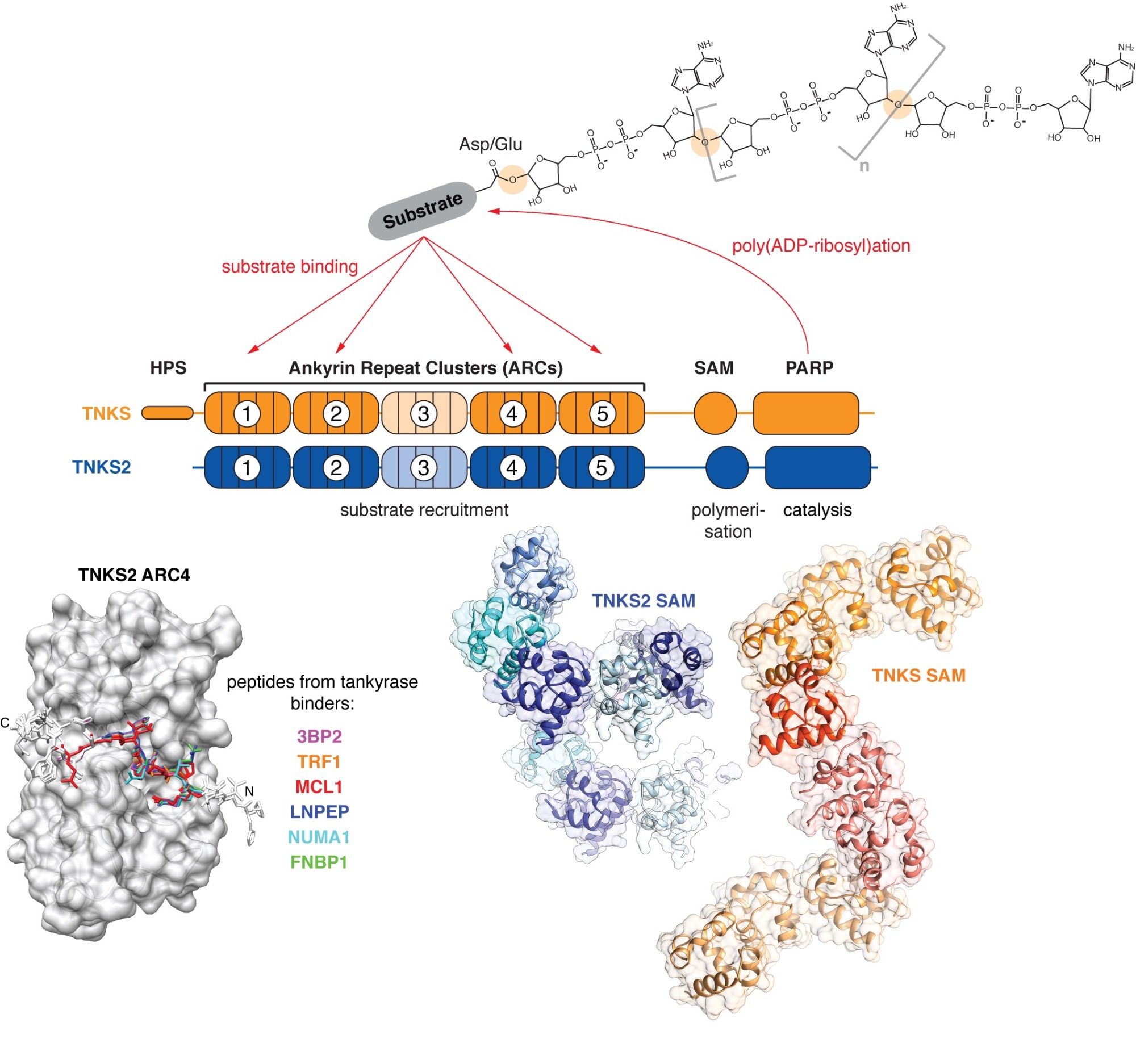 Tankyrase (TNKS, TNKS2) uses its ankyrin repeat clusters (ARCs) to recruit binding partners, many of which are also PARylated by tankyrase’s PARP domain. ARCs recognise degenerate peptide motifs found in many proteins. Our earlier work (Guettler et al., 2011) has revealed the substrate recognition mechanism and explained how the rare human disease Cherubism is caused. The sterile alpha motif (SAM) domain enables tankyrase polymerisation. We have revealed the mechanism of tankyrase polymerisation and shown that both ARCs and the SAM domain polymer fulfil essential scaffolding roles and are required for efficient substrate modification (Mariotti et al., 2016). (Images modified from Guettler et al., 2011; Mariotti et al., 2016 and Pollock et al., 2017)
Tankyrase (TNKS, TNKS2) uses its ankyrin repeat clusters (ARCs) to recruit binding partners, many of which are also PARylated by tankyrase’s PARP domain. ARCs recognise degenerate peptide motifs found in many proteins. Our earlier work (Guettler et al., 2011) has revealed the substrate recognition mechanism and explained how the rare human disease Cherubism is caused. The sterile alpha motif (SAM) domain enables tankyrase polymerisation. We have revealed the mechanism of tankyrase polymerisation and shown that both ARCs and the SAM domain polymer fulfil essential scaffolding roles and are required for efficient substrate modification (Mariotti et al., 2016). (Images modified from Guettler et al., 2011; Mariotti et al., 2016 and Pollock et al., 2017)
Molecular mechanisms of Wnt/beta-catenin signalling, telomere maintenance and their regulation by poly(ADP-ribosyl)ation
In a series of projects, we take a reductionist approach to study how large macromolecular complexes coordinate Wnt/beta-catenin signalling and telomere length homeostasis and how they are controlled by tankyrase-dependent poly(ADP-ribosyl)ation. We combine biochemical assays with cryo-electron microscopy and X-ray crystallography to uncover the detailed mechanisms governing the functions of these complexes and their regulation.
Besides uncovering fundamental mechanisms underlying stem and cancer cell function, we endeavour to understand the molecular basis of disease mutations and open up new opportunities for pharmacological intervention.
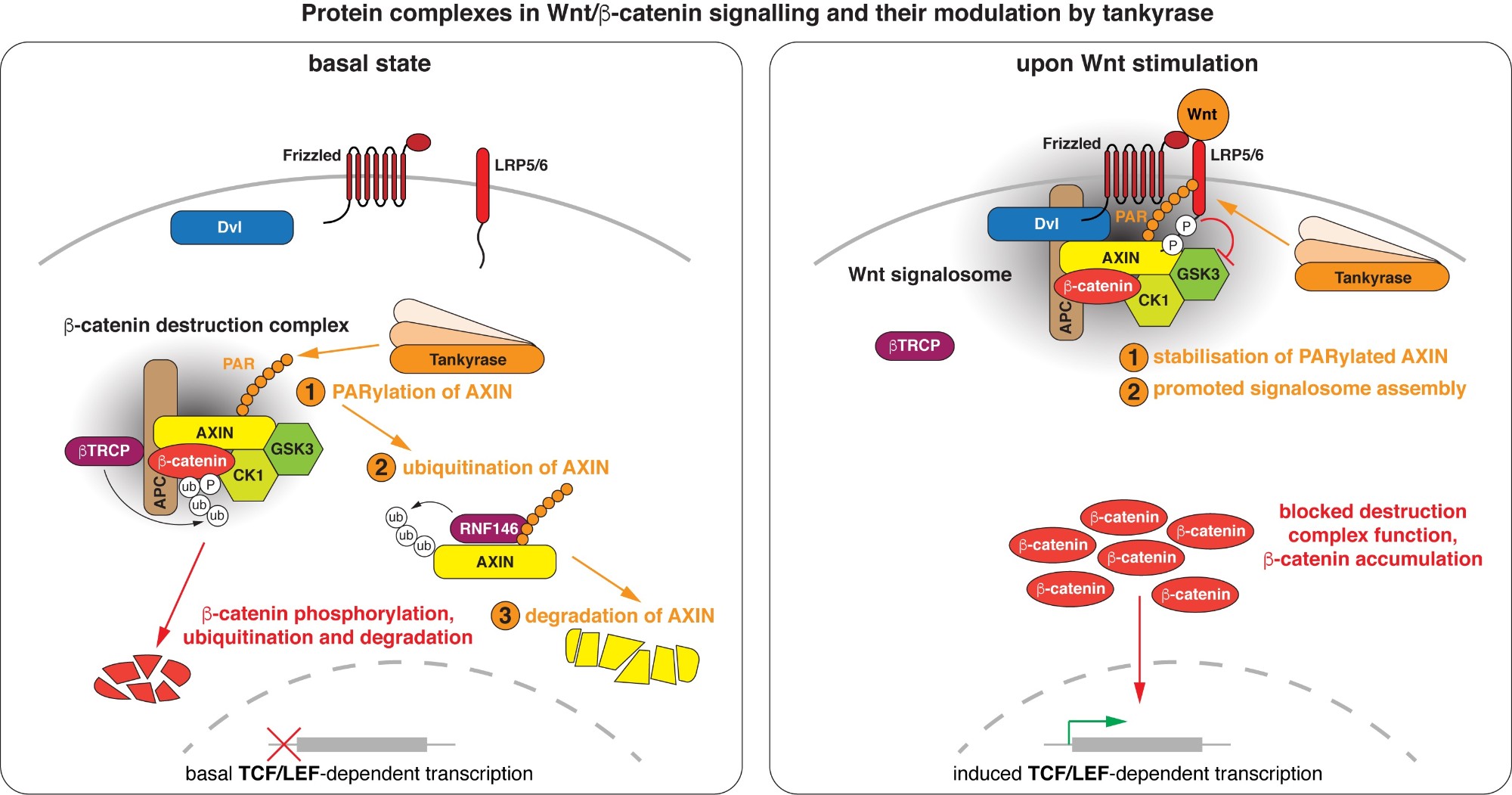
Wnt/beta-catenin signalling revolves around controlling the levels of the transcriptional co-activator beta-catenin. A multi-protein beta-catenin destruction complex captures cytoplasmic beta-catenin and limits its abundance by initiating its phosphorylation- and ubiquitination-dependent degradation. Notably, destruction complex function is impaired in the vast majority of colorectal cancer cases. Wnt stimulation remodels the destruction complex into a membrane-localised “Wnt signalosome” incapable of destabilising beta-catenin. Tankyrase controls the receptiveness of cells to incoming Wnt signals by PARylating AXIN, thereby destabilising the destruction complex or promoting Wnt signalosome formation. (Images modified from Mariotti et al., 2017)