a9511bcb659564f3a772ff0000325351.tmb-0874x492.jpg?sfvrsn=33c47f69_2)
Our article is written for a bioscience audience and offers guidance on best practice in chemical probe selection, evaluation and use.
I have previously posted about how small molecule chemical probes of high quality are crucial for investigating the function of proteins in cells and organisms – and also for validating them (or not) as drug targets. This applies to all areas of biomedical research – see articles by Stephen Frye and Mark Bunnage and colleagues.
My colleague Julian Blagg and I have today published a Perspective on choosing and using chemical probes in the journal Cancer Cell that is written specifically with the audience of biologists in mind.
Fit-for-purpose chemical probes – challenges and opportunities
There are numerous examples of how the use of fit-for-purpose chemical probes has led to important discoveries in biomedical research. The value of chemical probes is particularly well demonstrated in the field of cancer research.
A good case in point is the relatively recent rapid growth in our understanding of the biology and pharmacology of bromodomains that was triggered by the discovery of potent chemical probes such as JQ1 and I-BET and their closely matched inactive partner compounds used as controls.
Yet in my earlier blog I also drew attention to how loose standards in the selection and use of chemical probes are leading to serious errors in biomedical research studies.
To be effective as chemical probes, small molecule agents need to be cell permeable and to bind potently (i.e. strongly at low concentrations) to the desired protein target and modulate its function in the cell – as measured by direct target interaction and appropriate downstream biomarker changes. And they also need to bind selectively, meaning that they don’t interact with and modulate other cellular targets – or more realistically that they only affect an acceptable number of additional relevant proteins.
However, use of poorly selective, or otherwise unsuitably flawed – even frankly dreadful chemical compounds – is widespread. This is sloppy science and contributes to what is often referred as a ‘crisis’ in the reproducibility and robustness of biological findings.
Moreover, recent calculations have suggested that spending $150 on a poor quality, out-of-date chemical compound from a vendor catalogue, instead of buying a high quality chemical probe, can cost the scientific community billions of dollars. There are at least 200 ‘historic’ compounds that are often used and should be replaced with better probes.
So misuse of chemical probes is wasting scientists’ time and money – and in many instances is undoubtedly leading to delays in the discovery of much needed medicines.
I explained in my previous post how, in a Commentary article in the journal Nature Chemical Biology by Arrowsmith et al published in August 2015, an international panel of chemical biology scientists (of which I was a member) had issued a ‘call-to-arms’ – aimed at eliminating the use of substandard research tools in biomedical research and promoting best practice. That call was linked with the launch of a new community-based, 'TripAdvisor-style' online resource available at the Chemical Probes Portal.
The Chemical Probes Portal, search engines and continued use of flawed probes
The non-profit Portal works by offering online expert annotation and recommendations for use of chemical probes for particular molecular targets. These are provided by a Scientific Advisory Board (for full disclosure I am a member of this and a Board Director) – with about 400 probes assessed to date.
There’s no doubt that great progress has been made in discovering high quality tools for cancer biology and target validation. Unfortunately – two years on now from the publication of the Arrowsmith et al paper and the initial launch of the Chemical Probes Portal – it is abundantly obvious that bad practice in the selection and use of chemical probes is still very widespread in biomedical research, including numerous, continuing high profile examples in cancer.
It’s clear that biologists commonly choose chemical probes based on querying search engines such as Google – which will lead them to vendor catalogues that provide variable levels of information, do not prioritize probes based on quality, and sometimes recommend the same compound as a probe for different protein targets.
Alternatively, use of search engines like Google Scholar will return as top hits publications that are the most highly cited, but that also describe the oldest chemical probes. Such searches are less likely to find the best, usually more recent tools. For example, when Chemical Probes Portal staff looked at 10 compounds, randomly selected from the 200 no longer recommended historical probes listed on the Portal website, they found that since 2016 these past-their-sell-by date reagents have been used in 2,090 publications.
A specific illustration is the still very frequent use of one of the above historical compounds, LY294002 – an initially valuable early inhibitor of the phosphoinositide 3-kinase lipid kinases (PI3 kinase) that was originally described in 1994. Although a useful ‘pathfinder probe’, LY294002 exhibits only weak, micromolar potency for PI3 kinases and through chemoproteomic studies it was subsequently found to be active against numerous members of the PI3 kinase family, and also other unrelated proteins including bromodomains.
LY249002 has been cited in over 30,000 publications; moreover despite its poor potency and selectivity and its supersedence by several superior compounds as chemical probes for PI3K, a recent search for LY294002 on Google Scholar returned 1,190 publications for the year 2016 alone and this now outdated and flawed probe continues to be sold by many commercial vendors.
Our new Perspective in Cancer Cell
It’s clear then that we need to find a way make things change and especially influence behaviour of biological research community which is the main user group for chemical probes. This is why Julian Blagg and I have written our Perspective in way that we hope will get the message out to biologists.
As we say in the Abstract of the Perspective:
‘Small-molecule chemical probes or tools have become progressively more important in recent years as valuable reagents to investigate fundamental biological mechanisms and processes causing disease, including cancer. Chemical probes have also achieved greater prominence alongside complementary biological reagents for target validation in drug discovery. However, there is evidence of widespread continuing misuse and promulgation of poor-quality and insufficiently selective chemical probes, perpetuating a worrisome and misleading pollution of the scientific literature. We discuss current challenges with the selection and use of chemical probes, and suggest how biologists can and should be more discriminating in the probes they employ.’
Despite the efforts so far within the chemical biology community, we point out that we have been guilty of: ‘largely preaching to the choir [meaning chemical biology specialists] and failing to connect to a really critical audience: namely, the wider cancer biology community who rely upon small-molecule tool compounds, often in harness with biological reagents, to interrogate cancer cell biology and who frequently draw important and highly impactful biological interpretations, whether correct or misleading, from such studies’.
Examples of misleading findings with flawed probes
A topical example that we highlight in our Perspective is the initially erroneous discovery and validation of the proposed target MTH1 in cancer. MTH1 has a role in breaking down damaged metabolites called nucleotides in cells and thus preventing them from being incorporated into DNA – and was first published as a cancer target in very high profile publications in the journal Nature.
Small molecule agents that were originally used to validate MTH1 include compounds TH287 and TH588 as well as S-crizotinib. We discuss in our Perspective the elegant publication from AstraZeneca scientists that identifies three different chemical series of potent and highly selective chemical probes that clearly inhibit MTH1 in cancer cells but despite this have no therapeutic effect on cancer cells.
Furthermore, the same article shows that neither small interfering RNA (siRNA) reagents that deplete MTH1 nor CRISPR-mediated removal of MTH1 had any beneficial effect of cancer cells, pointing to off-target activity with the original siRNA reagent as well the chemical compounds used.
Furthermore, the blogger Derek Lowe has just a couple of days ago updated this story by discussing a new publication from researchers at Bayer who discovered BAY-707, yet another highly potent and selective inhibitor of MTH1, and found it to have no therapeutic effect on cancers cells. Hence at this stage the balance of opinion strongly indicates that MTH1 is not a valid target for cancer treatment.
One piece of evidence in the AstraZeneca study that was particularly critical in invalidating the initial chemical probes was the demonstration that both TH287 and S-crizotinib killed cancer cell lines lacking MTH1 – and subsequent protein screening work showed that the binding of TH287 and TH588 to tubulin is responsible for their cytotoxic effects.
We provide in the Perspective several other examples of how the close integration of orthogonal chemical and biological tools can be very powerful, as in the case of studies on SWI/SNF chromatin remodelling complex components, the transcription factor HIF2α and the Jumonji family of histone lysine demethylases. In addition, we describe cautionary tales of the problems arising with uncritical use of claimed chemical probes for proteins including poly ADP ribose polymerases (PARPs; for which a flawed PARP compound progressed to the clinic and failed); the molecular chaperone HSP70; KRAS-regulated autophagy; and pan-steroid receptor co-activators.
Choosing and using wisely – caveat emptor
We discuss how a gold-standard test to validate the functional on-target response to a chemical probe is to demonstrate reversal of the cellular effects of a proposed small molecule probe compound by mutation in the protein target that abrogates compound binding.
Another useful technique is to engineer the target to interact with chemical probes not recognized by the natural (so-called) wild-type protein. An additional approach now becoming common is to determine the effects of the chemical probe in cells where the proposed protein target has been removed by CRISPR technology.
We point out that although ‘Small molecules are from Mars, biological tools are from Venus’, they are nevertheless part of the same overall universe, providing orthogonal and complimentary approaches to understanding biology and target validation – a very powerful, multidisciplinary and essential toolkit for modern biomedical research.
Also in our Perspective we highlight and explain an important aspect of target binding selectivity that is rarely articulated in discussion of chemical probes – that it is absolutely to be expected that most small molecules will generally interact with multiple biological targets in cells and organisms. By contrast, biological reagents, for example siRNA oligonucleotides and antibodies, are intrinsically more likely than small molecules to bind selectively to the desired biological target as a result of the greater breadth, complexity and thus specificity of their combined intermolecular interactions.
Of course there are also major problems with the use of insufficiently selective biological reagents and greater rigour in their use is important too – as elegantly discussed recently by Bill Kaelin – but biologists need to be even more critical in their use of small molecule probes because their smaller size and lower complexity means that at least some degree promiscuity is likely to be the rule rather than the exception. This tendency can be mitigated by careful design and optimization of the probe – but even then rigorous and broad experimental testing for selectivity is essential.
Indeed, we strongly advise the maxim of caveat emptor – let the buyer beware! – when choosing and using chemical probes for biological exploration and target validation.
In discussing the challenge of selectivity, we illustrate how the off-target effects can range from an interaction with one or two proteins – perhaps but necessarily related to the target of interest – through binding to tens of other targets, all the way to the extreme end of unacceptability where compounds are ‘frequent hitters’ or ‘chemical imposters’ that have totally unacceptable features like indiscriminate chemical reactivity, aqueous insolubility and self-aggregation that make them worthless for biological research.
Hard to believe, but there are even isolated examples of vendors supplying the incorrect chemical compound and routine checking for evidence of authenticity is advisable. Related to this, in our Perspective we call for further efforts in the community to eliminate the especially egregious behaviour of publishing biological results without disclosing compound structures – which of course means that the suitability of a probe cannot be assessed, nor can the claims be independently checked. Reviewers of submitted papers and grant applications as well as journal editors should be especially vigilant about this.
We recognize that for many if not most biologists these considerations of the selectivity of chemical probes are not part of their training or expertise. They may not have ready access to advice from chemical biology or medicinal chemistry colleagues. And they may find articles in the specialist chemical biology literature off-putting and full of jargon – as most scientific disciplines are.
Useful tools for biologists
So in our Perspective we provide what we hope will be useful tools for biologists using chemical probes. Firstly, we include as Box 1 a Glossary so that that any specialist terminology that cannot really be avoided is not too much of a turn-off.
We provide in Box 2 a comparison of the desired selectivity profiles of chemical probes with those of approved drugs – making the point that in comparison to drugs, chemical probes generally need to be even more selective than drugs so that probes can be used with confidence to modulate the intended target of interest.
In Box 3 we summarize the factors that determine the fitness and quality of chemical probes and in Figure 2 we present an overview of ‘Dos and Don’ts’ for their selection and use. In particular, we strongly recommend taking a routinely sceptical approach, including the use of orthogonal chemical and biological reagents; the use of at least two different chemical series (chemotypes) of probe along with inactive control compounds; demonstration of potency and selectivity; and obtaining evidence for selective target engagement and modulation in cells (e.g. using the Pharmacological Audit Trail).
We advise (on page 13 of the Perspective) strongly against a common and dangerous practice, which is to expose cells with ever increasing concentrations of a chemical probe until a desired cell effect (phenotype), usually cell death, is seen – and then attributing this phenotype to the specific effect of the probe on the protein target under investigation.
Higher probe concentrations increase the likelihood of off-target effects and the general range that should not be exceeded is 10-20 micromolar to minimize non-specific effects. Accompanying biomarker evidence of target modulation is also important.
Alongside the general guidance provided in our Cancer Cell Perspective, we strongly recommend the use of the Chemical Probes Portal for expert advice and ratings for specific probes and targets.
Avoiding using a defective global positioning/satellite navigation system – a new call-to-action
We liken the provision of advice on the selection and use of chemical probes to ensuring the biological researcher avoids being equipped with the equivalent of a defective global positioning/satellite navigation system, as illustrated in the cartoon below:
Download a larger version of Professor Julian Blagg's cartoon (PDF, 51KB)
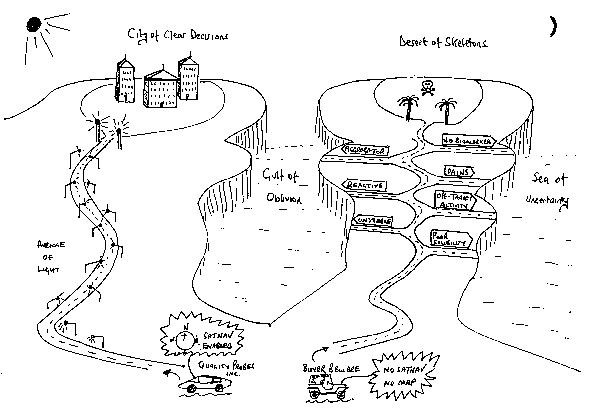
Caption: The right way and wrong way with chemical probes
We finish the Perspective with the following strong new call-to-action:
‘We need to maximize the promise and minimize the peril of chemical probes… and this requires the broad research community to use high-quality chemical probes that have been critiqued with equivalent rigor to biological reagents. It is time to put our house in order – and biologists as well as chemists have an important responsibility to do so.’
Acknowledgements
I’m grateful to my colleague and joint senior co-author of our Cancer Cell Perspective Professor Julian Blagg for his excellent collaboration and insights. We developed the content of the Perspective very much in partnership. I also thank Julian for drafting the cartoon illustration.
In addition, I thank many colleagues and collaborators for helpful discussions and input, including the anonymous reviewers of the Perspective, and those in the field whose outstanding work we have built upon.
comments powered by